3 min read
October 23, 2025
Your Launch Date Just Moved Up
When approvals come early, we help biotech teams stay launch-ready, no heroics needed.
There is a quiet paradox in biotech launch planning: everything revolves around a PDUFA date. Until it doesn't. Approval can arrive early, like a surprise houseguest who texts “I’m outside” from your driveway. Meanwhile, your website is a wireframe, your claims live in five versions of a Word doc, the MLR queue is longer than Soviet line to buy tinned fish, and your CRM is set up for “future state” rather than Tuesday.
No villains here; just reality. Pharma's operating model is built around PDUFA, a date that is both critical and unreliable. That's the gap AIMBio was built to close using “ready no matter what” basics that survive the unpredictability. Here's the truth: launch readiness is a timing problem disguised as a content and channel problem. When timing is uncertain, readiness has to be continuous.
The launch readiness problem isn't lack of strategy or talent. It's the collision between regulatory timing and commercial reality: processes that can't magically speed up, dependencies that multiply under pressure, and a runway measured in quarters while the market expects precision to the week.
What Breaks When Approval Comes Early
The same three things, every time:
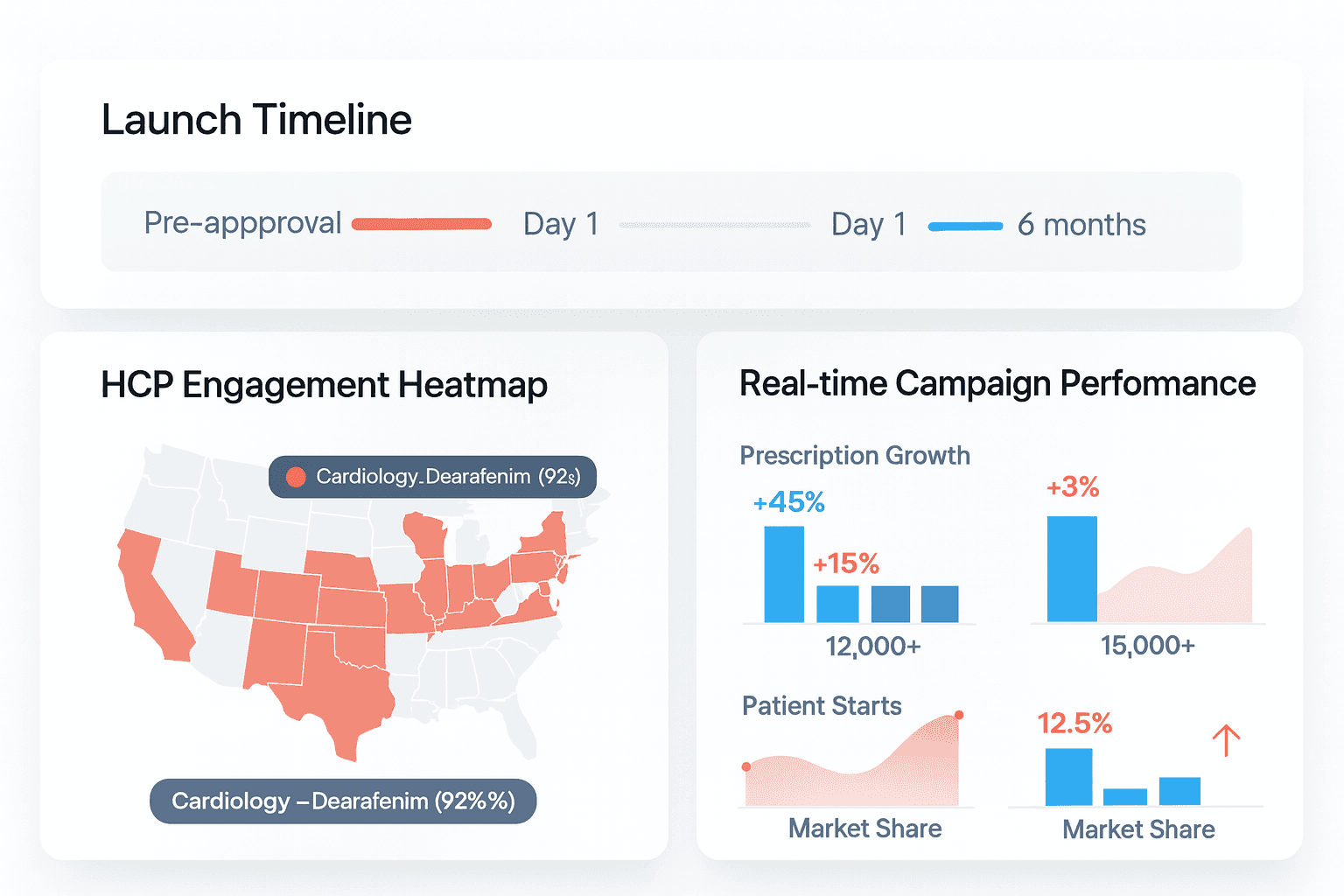
Findability. Clinicians and patients search, AI overviews surface answers, and your site either appears with clarity; or your competitor’s does.
Reviewability. If claims, references, and modular content weren't prepped for reuse, MLR becomes a bottleneck right when speed matters most.
Usability. Field and digital need a shared cadence that a small team can actually run.
When those fundamentals aren't installed in advance, you end up pouring a foundation while dinner guests are arriving.
What Readiness Actually Looks Like
None of this requires a sermon. It requires sequencing. Before approval, even before LR3, you can quietly put in the plumbing: a site information architecture built around real HCP and patient questions, schema and technical SEO that make content legible to both humans and machines (yes, those AI friendly ones), a claims architecture that snaps into review packets without starting from zero, and an omnichannel rhythm sized for 25 reps, segmenting your HCP target list into more than just 5 regions. It's not flashy, but it's the difference between “we launched” and “we landed.”
People sometimes ask how this compares to enterprise platforms. Different jobs. There's a moment for global transformation; there's a moment for getting the right pieces live before the market rings the bell. AIMBio is built for that second moment: the high-leverage window when “minimum viable launch” isn't a buzzword, it's a lifeline.
We design for what your team must do this week, and we architect so it won't paint you into a corner next quarter. That means building assets that are preapproved and reusable, a site that's both compliant and searchable, and a measurement loop that tells you which action to take next. Not a month from now, but next Monday.
The Bottom Line
Biotech doesn't stumble at launch because no one thought hard enough. It stumbles because the calendar moved and the basics weren't ready yet. AIMBio exists to set this up quietly, quickly, and in a way your team can sustain. So when the FDA fax comes early, you don't need heroics; you just hit GO.
If you're inside that six-to-twelve-month window and any of this feels uncomfortably familiar, send us your date, your current state and stack (even if it's Google Drive + hope), and the three things keeping you up at night. We'll tell you what needs to be live Tuesday, and what can wait for the post-launch debrief.
Launch Day 1. Build to Scale.
AI‑powered digital launch readiness: we compress months of work so your campaigns and infrastructure are live at approval and ready to grow with your portfolio.
10x Growth
Product adoption accelerated by digital innovation
15+ Years
Building digital engines for biotech and pharma
20+ Brands
Launches across oncology, rare disease & specialty